PreludeDx is a leading personalized breast cancer diagnostics company dedicated to serving breast cancer patients and physicians worldwide. Pulse 2.0 interviewed PreludeDx President and CEO Dan Forche to learn more.
Dan Forche’s Background

Dan Forche has over 25 years of diagnostics experience with 20 years focused in oncology, working for large companies such as Abbott Labs and LabCorp as well startup companies in which Forche was instrumental in launching novel testing.
Forche is passionate about advancing personalized care. He explained that “Over the years, several of my relatives were diagnosed with cancer and this sparked my interest in both the oncology space and personalized medicine. I was introduced to oncology very early in my career while working at Abbott providing testing for unique tumor markers. Subsequently, I was part of the team that introduced one of the first precision medicine tests, Her2neu for invasive breast cancer at Ventana/Roche.”
“It is estimated that precision medicine testing will grow from $66.22 billion in 2021 to $175.6 billion by 2030, with oncology being the most prominent disease state benefiting from precision medicine.”
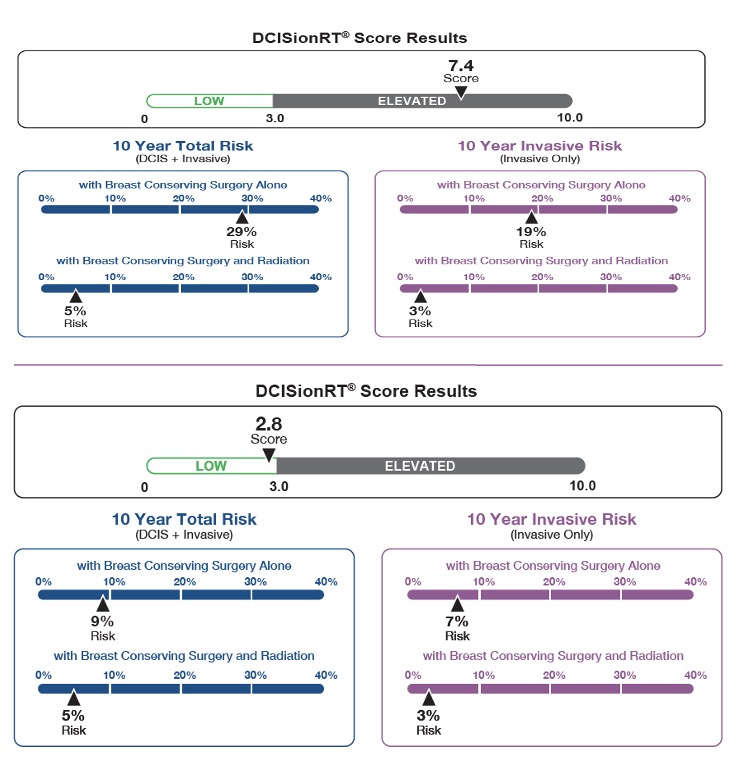
PreludeDx is a leader in molecular diagnostics and precision medicine for early-stage breast cancer focused on bringing novel tests to the clinic to help patients and their physicians make personalized treatment decisions. The current standard of care for ductal carcinoma in situ (DCIS), also known as stage zero breast cancer, is to provide adjuvant radiation therapy following breast conserving surgery. While only 20-25% of these women are expected to benefit from adjuvant radiation therapy, more than 80% are typically treated with radiation therapy. This approach occurs because until recently, doctors only had access to clinical and pathological features, such as tumor grade, tumor size, and margin status, to identify who to radiate instead of the patient’s own biology.
The DCISionRT test provides a quantitative personalized result that enables patients and their physicians to personalize treatment decisions, minimizing over- and under-treatment and improving patient quality of life.”
Formation Of PreludeDx
How did the idea for PreludeDx come together? “Prelude was born out of new biomarker data shared on DCIS in 2006 at the San Antonio Breast Cancer Symposium by researchers at the University of California San Francisco (UCSF),” Forche shared. “We licensed the initial biomarker technology from UCSF and extended the discovery work before the company was officially founded in 2009. PreludeDx’s goal has always been to bring transformative testing to early-stage breast cancer patients. For the next several years, PreludeDx continued to develop the technology to biologically assess patient risk and radiation therapy benefit. The DCISionRT test was subsequently developed and extensively validated prior to clinical launch in 2017.”
Favorite Memory
What has been Forche’s favorite memory working for PreludeDx so far? “One of my fondest memories is the impact of our first clinical DCISionRT test and what it did to improve patient care. The patient informed her breast cancer surgeon that she did not want radiation at all and was not going to do it. The surgeon shared that there was a new company with a new test that could help to quantify her personal risk of recurrence after breast-conserving surgery and predict whether or not she would benefit from radiation therapy. PreludeDx ran the test, and the result came back that the patient was at an elevated risk and would benefit significantly from radiation therapy,” Forche reflected. “So, based on this new novel information, the patient who was initially against RT (radiation therapy), decided that based on her personalized DCISionRT results, she would get RT. This was really powerful to see how new and better information changed the patient’s decision.”
Challenges Faced
What are some of the challenges Forche faced in building the company and has the current macroeconomic climate affected the company? “Funding is always a challenge for an emerging growth company. We were fortunate to raise $20 million in March 2022 as our current and new investors saw the opportunity in precision medicine that PreludeDx is bringing to patients and physicians throughout the US and worldwide.” Forche continued, “Medicare reimbursement is widely acknowledged as difficult when bringing new innovations to the market; however, we have made significant progress on that front and earlier this year we received Advanced Diagnostic Laboratory Test (ADLT) status from the Centers of Medicare and Medicaid Services (CMS). PreludeDx is only the eighth company to receive ADLT distinction, which demonstrates that our test provides novel clinically relevant information to patients not available elsewhere.”
Core Products
What are PreludeDx’s core products? Forche replied: “DCISionRT combines the latest innovations in molecular testing to assess a woman’s individual tumor biology along with other pathologic risk factors and provides a personalized recurrence risk, identifying a woman’s risk as low or elevated, following breast-conserving surgery with and without adjuvant radiation. The DCISionRT test is currently being utilized by over 1,500 physicians and top cancer centers throughout the U.S. and worldwide.”
DCISionRT’s intelligent reporting provides a woman’s recurrence risk after breast-conserving surgery alone and with the addition of radiation therapy. In turn, this new information helps patients and their physicians to make more informed treatment decisions.
A study published in the Annals of Surgical Oncology demonstrates that the use of DCISionRT led to a change in RT treatment recommendations in 42% of patients with DCIS compared to traditional clinical and pathological features.
Women and their physicians now can have greater peace of mind when making a personalized decision to add or omit radiation therapy after breast-conserving surgery.
The highest level of clinical evidence from landmark clinical trials repeatedly demonstrates clinpath fails to ID patients who can safely omit RT. Clinpath is defined as the clinicopathologic features such as patient age, nuclear grade, and tumor size that have been traditionally used to determine a patient’s level of risk. DCISionRT analyzes a patient’s specific tumor biology and predicts % disease recurrence with surgery alone or surgery plus RT and provides a quantitative, personalized test result that contributes to making the most informed treatment decision.
Evolution Of PreludeDx’s Technology
How has PreludeDx’s technology evolved since launching? “When DCISionRT first launched, the test could answer the questions ‘which patients will have no to minimal benefit from radiation therapy’ and ‘which patients will have significant benefit from radiation therapy’ following breast-conserving surgery. This year, we introduced our residual risk group which identifies patients who remain at significant risk after breast conserving surgery and radiation therapy,” Forche revealed.
Significant Milestones
What have been some of PreludeDx’s most significant milestones? “Receiving ADLT status from CMS was a major milestone for PreludeDx and confirms the clinical value and unique nature of the DCISionRT test. The DCISionRT test is the only test that can predict radiation therapy benefit for women diagnosed with DCIS and this new information improves shared decision-making between patients and their treating physicians. PreludeDx is only the eighth company to receive Advanced Diagnostic Laboratory Test distinction,” Forche pointed out. “In September 2022, we published data demonstrating that DCISionRT is the first and only test to identify DCIS patients with unexpectedly high residual risk after surgery and radiation. DCISionRT is also the only DCIS test validated with peer-reviewed published level 1b evidence.”
Customer Success Stories
Upon asking Forche about customer success stories, he replied: “We are finding immense support for DCISionRT among surgeons, radiation oncologists, and patients. Across the board, these groups value the quantitative data for added confidence, shared decision-making, and improved patient outcomes. Once physicians begin using the test, they come to rely on the data and feel confident in the fact that they are treating patients with radiation who will truly benefit and enabling low-risk patients to potentially avoid radiation. On the patient side, we hear multiple stories on how DCISionRT test results changed a patient’s mind about their course of treatment.”
Funding
PreludeDx raised $20 million in March 2022 to advance growth initiatives and develop its precision medicine portfolio.
“We have also secured ADLT distinction from CMS and multiple agreements with health plans, including MediNcrease, Medical Cost Containment Professionals, Galaxy Health Network, and Three Rivers Provider Network. The funding and the new reimbursement from Medicare will allow the company to invest in more physician and patient advocates and reach more women with this needed technology across the globe,” Forche revealed.
Total Addressable Market
What total addressable market size is PreludeDx pursuing? “We define our global early-stage breast cancer market at over 1 billion and growing,” Forche assessed.
Differentiation From The Competition
What differentiates PreludeDx from the competition? Forche affirmed:
DCISionRT is the only test that provides both the risk of breast cancer recurrence after breast-conserving surgery and the risk of recurrence after BCS and adjuvant radiation therapy. The DCISionRT test measures the protein expression of seven genes that were specifically selected for assessing DCIS biology and combines these biomarkers with four clinicopathologic factors using a non-linear algorithm to better interpret complex biological information.
DCISionRT has been clinically validated to identify a true low-risk group of patients who have a low risk of recurrence after breast-conserving surgery and have minimal to no benefit from adjuvant radiation therapy or you would need to treat 100 patients in our low risk group to benefit 1. The test has also been clinically validated to identify patients with higher risks of recurrence after breast-conserving surgery who have different recurrence risks remaining after treatment with adjuvant RT.
DCISionRT has been determined to be cost-effective by an independent publication from top cancer centers and is easily incorporated into routine clinical practice with a turnaround time of about 5 days. In addition, the test has been validated to change RT treatment recommendations for about 40% of patients in a large prospective clinical study.
The DCISionRT test has been adopted by top academic cancer centers and community centers through the US and worldwide to help guide personalized treatment for women diagnosed with DCIS.
Future Company Goals
What are the company’s future goals? “We plan to expand our DCIS molecular testing portfolio into early-stage invasive breast cancer, as well as other cancer indications and we will continue to grow our global footprint,” Forche concluded.


