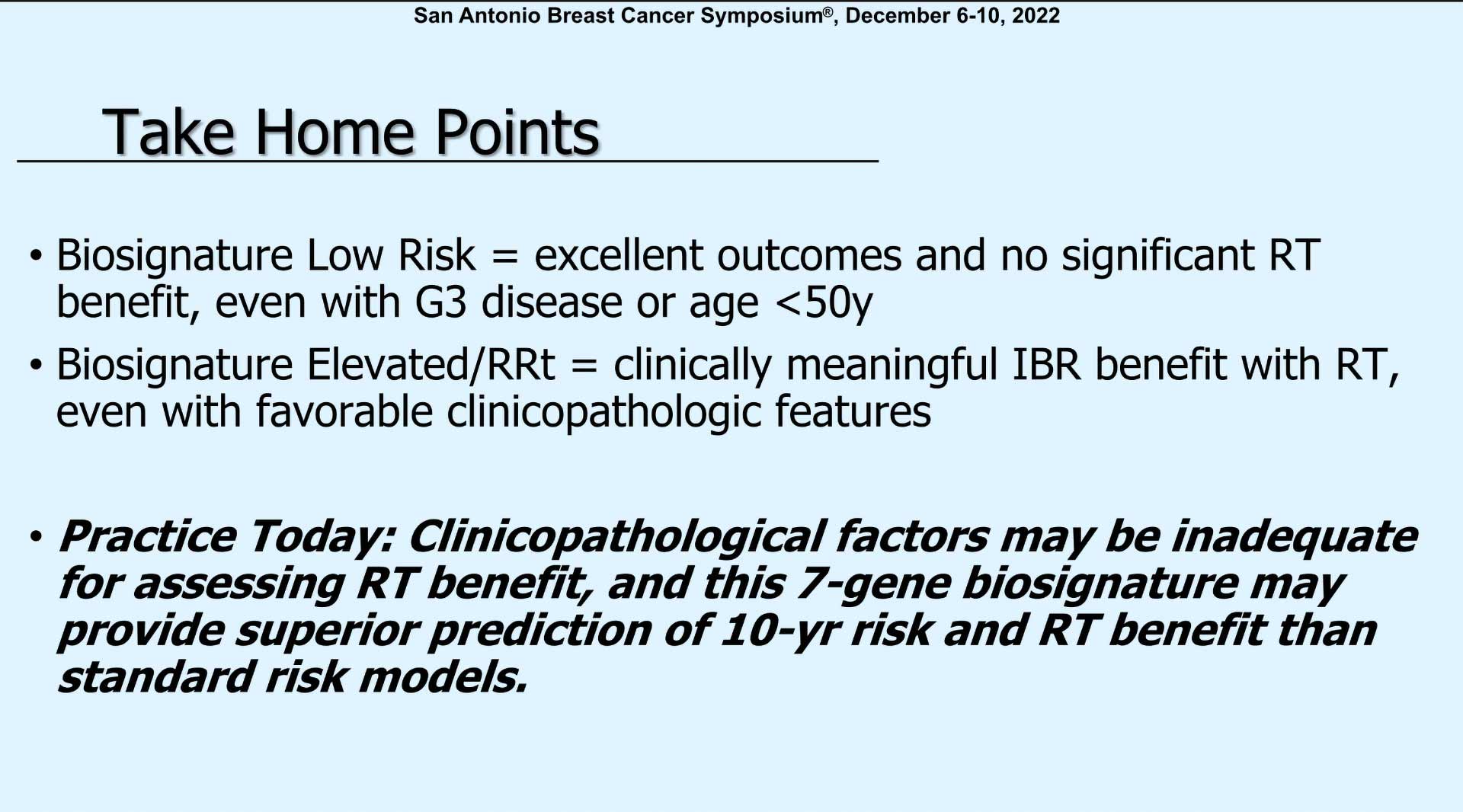
Dr. Plitcha concluded her discussion by saying, ‘So, in your practices today, clinical pathologic factors may not be adequate for assessing radiation benefit. And the 7-gene biosignature may provide superior prediction for 10-year risk and radiation benefit than standard risk models.’
Dr. Plitcha led a discussion at the Spotlight Session titled, ‘Local Regional/Management of the Axilla’ at the 2022 San Antonio Breast Cancer Symposium. PreludeDx’s poster titled, ‘7-gene predictive biosignature improves risk stratification for breast ductal carcinoma in situ patients compared to clinicopathologic criteria’, was among the posters discussed.

Dr. Plitcha began her discussion with the recognition that, ‘DCIS (ductal carcinoma in situ) is a very popular topic being studied by many collaborators at my own institution’.
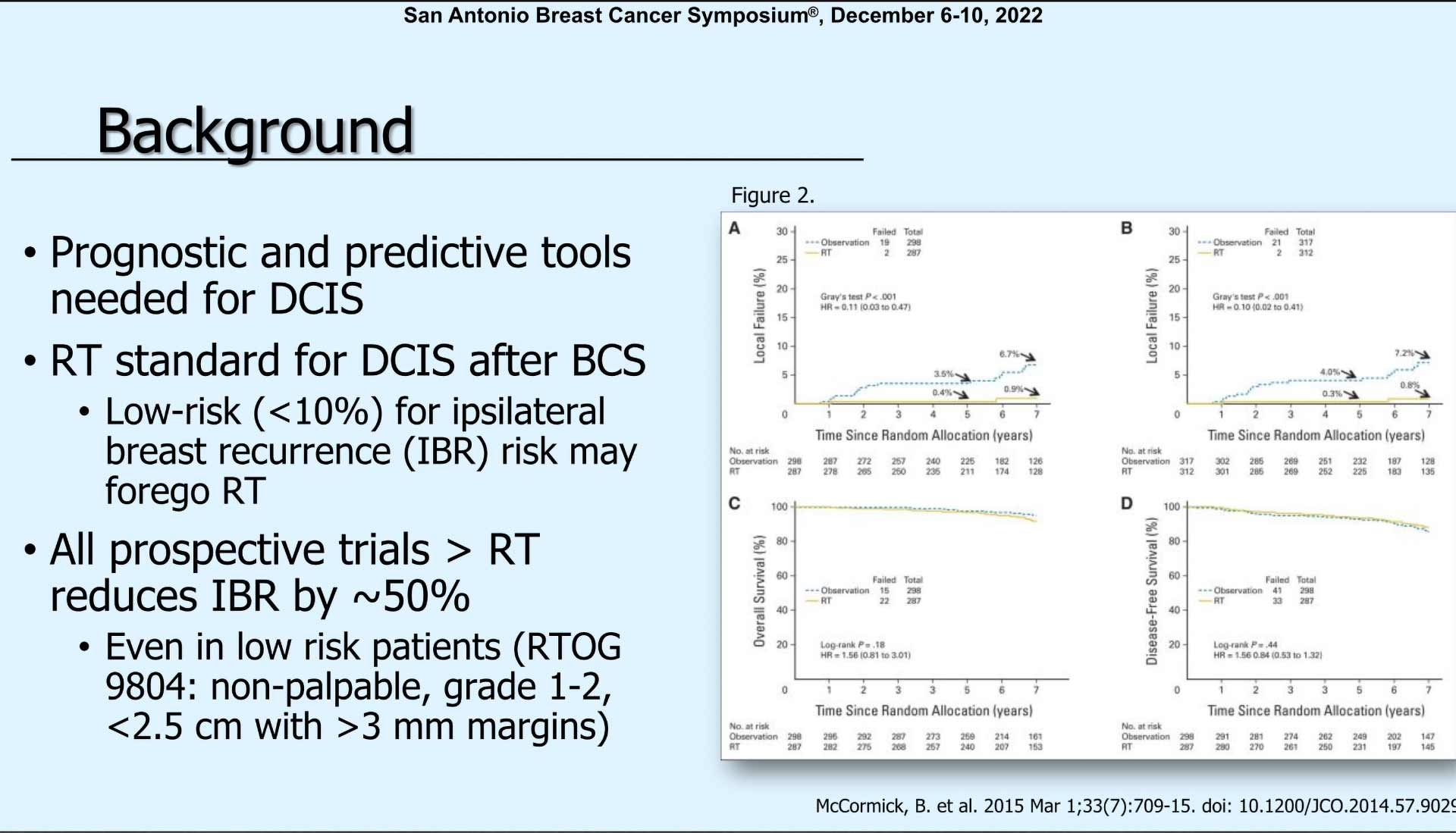
“We know there are some prognostic and predictive tools that are needed in this population to determine what is actually the most appropriate treatment for these patients. We know from other studies that radiation is the standard for DCIS because it has been shown to reduce the ipsilateral breast recurrence rate by about 50% in most prospective studies.”
“And this has even been shown to be true in low-risk patients, such as those in RTOG 9804, where low-risk patients are defined as non-palpable, grade one to less than 2.5 centimeters of DCIS with greater than three-millimeter margins.”
“You can see in this study published in 2015, exactly that. In A and B, you can see higher local failure rates for patients who did not have radiation. But in the bottom two graphs overall survival and disease-free survival were equivalent whether they had radiation or not.”
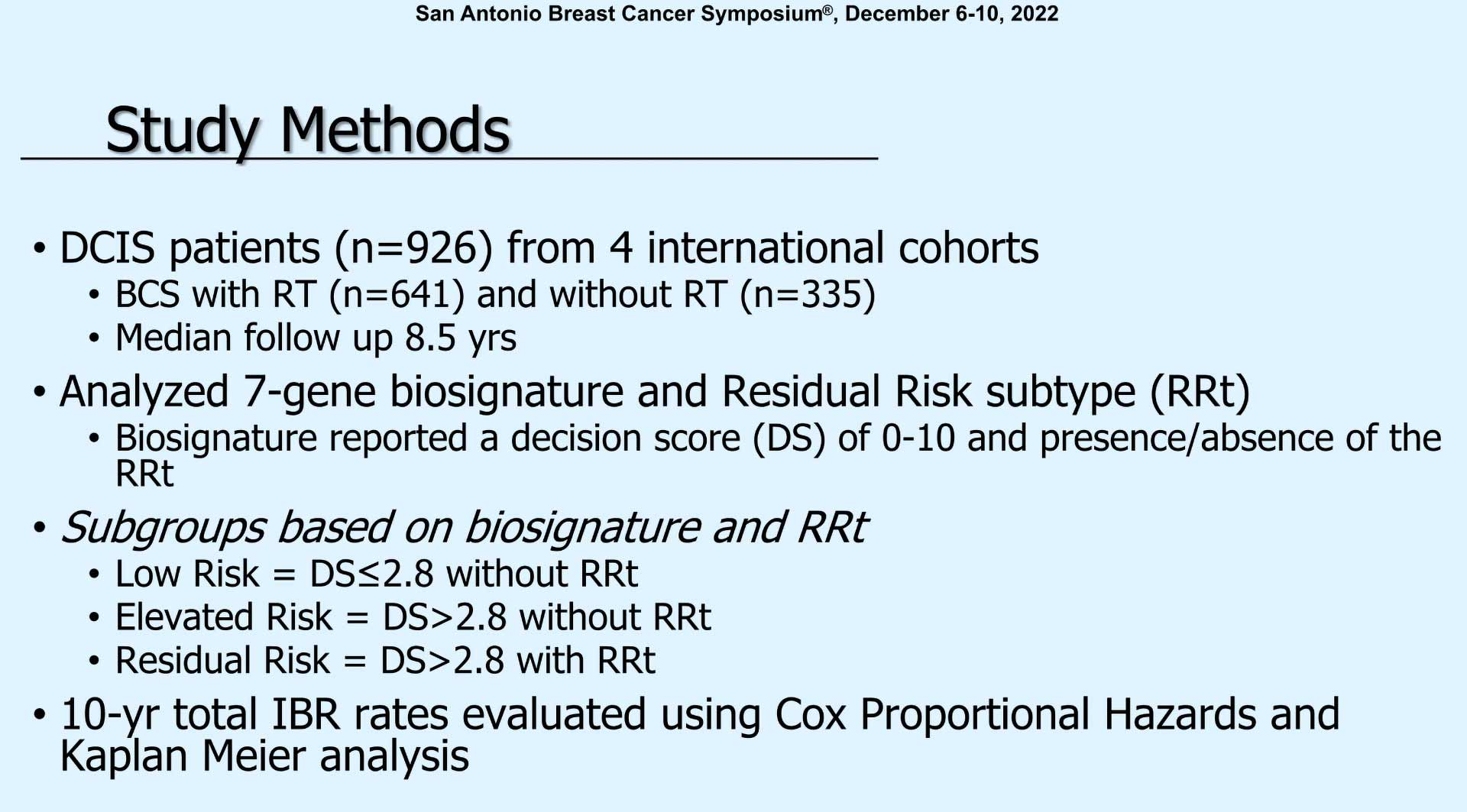
“This study aim was to analyze a cohort of women with DCIS who had breast conservation and determine if a biosignature could identify a subset of women who do not benefit from radiation and evaluate the biosignature in patients meeting low-risk or high-risk criteria. For this study, low-risk was defined by favorable, clinical pathologic criteria such as age greater than 50, grade 1 or 2, and the RTOG-9804 like disease, which was grade 1 or 2 and screen detected.”
“The DCIS patients included about 1,000 (patients) from 4 international cohorts. Median follow-up was actually quite good at 8 ½ years. They analyzed 7-gene biosignature and residual risk type which has been previously reported. And the biosignature reported a Decision Score of zero to ten and the presence or absence of this residual risk subtype. So essentially there were three subgroups. The low-risk which was a Decision Score less than 2.8 and without the residual risk subtype (RRt). Elevated risks with a Decision Score of greater than 2.8 without RRt. And the residual risk which was Decision Score of greater than 2.8 with RRt. And they looked at 10-year total ipsilateral breast recurrence rates.”
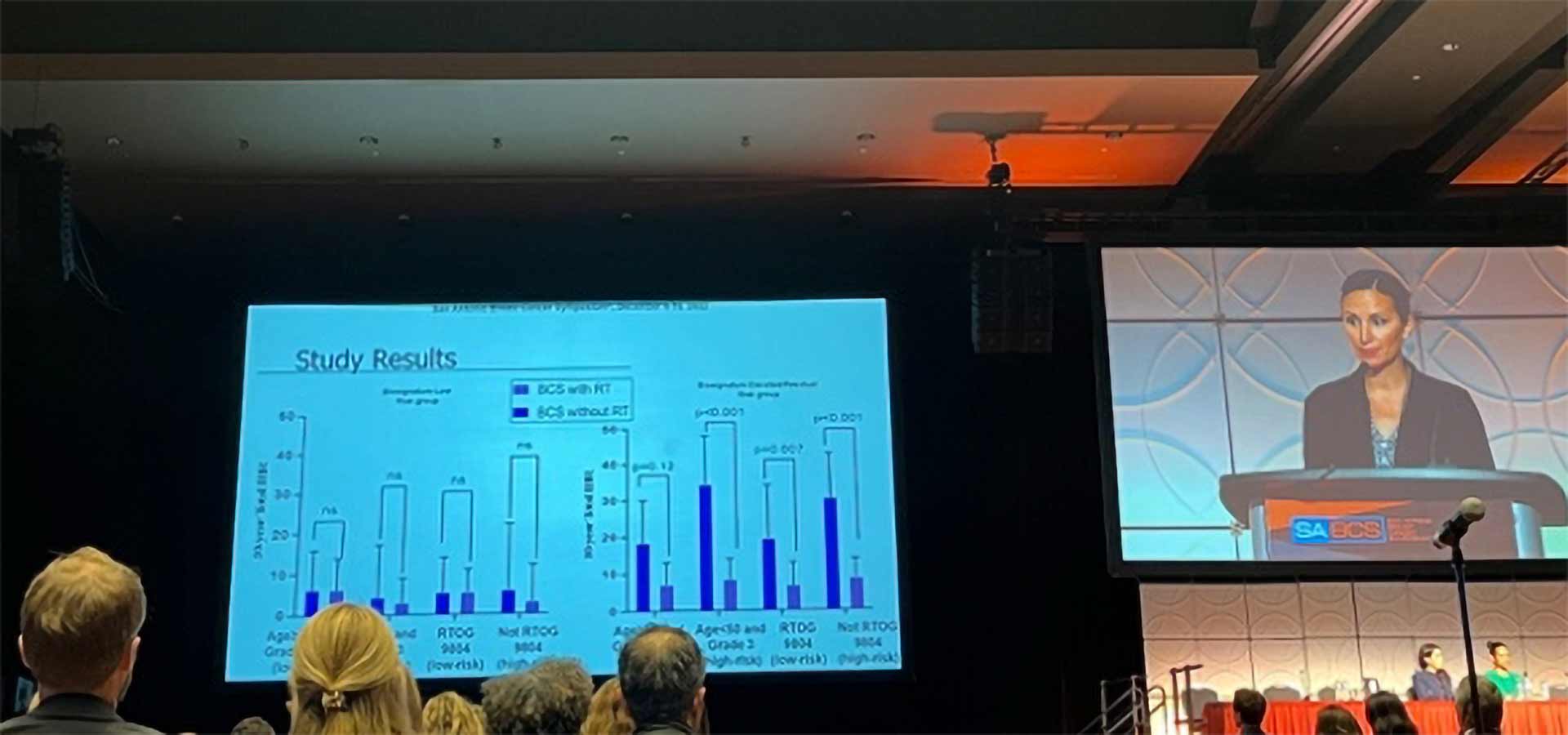
“You can see in the graph on the left we have the patients who had a low biosignature risk group. And then they looked at within those who had a low biosignature based on their clinical pathologic characteristics. You can see that there was a nonsignificant difference in those who did and did not receive radiation no matter what the clinicopathologic characteristics – older age, younger age, higher grade, lower grade. Whether they were like RTOG 9804 or not. And there was no statistical difference in the low-risk biosignature group. However, if you look at those who had a high, elevated or residual risk biosignature score, there was a statistically significant difference in all groups favoring radiation.* And of note, there is a typo here this .12 should be .012. So again, all 4 of these groups were statistically different. Even in those who had low-risk clinicopathologic criteria, there was a benefit to radiation.”
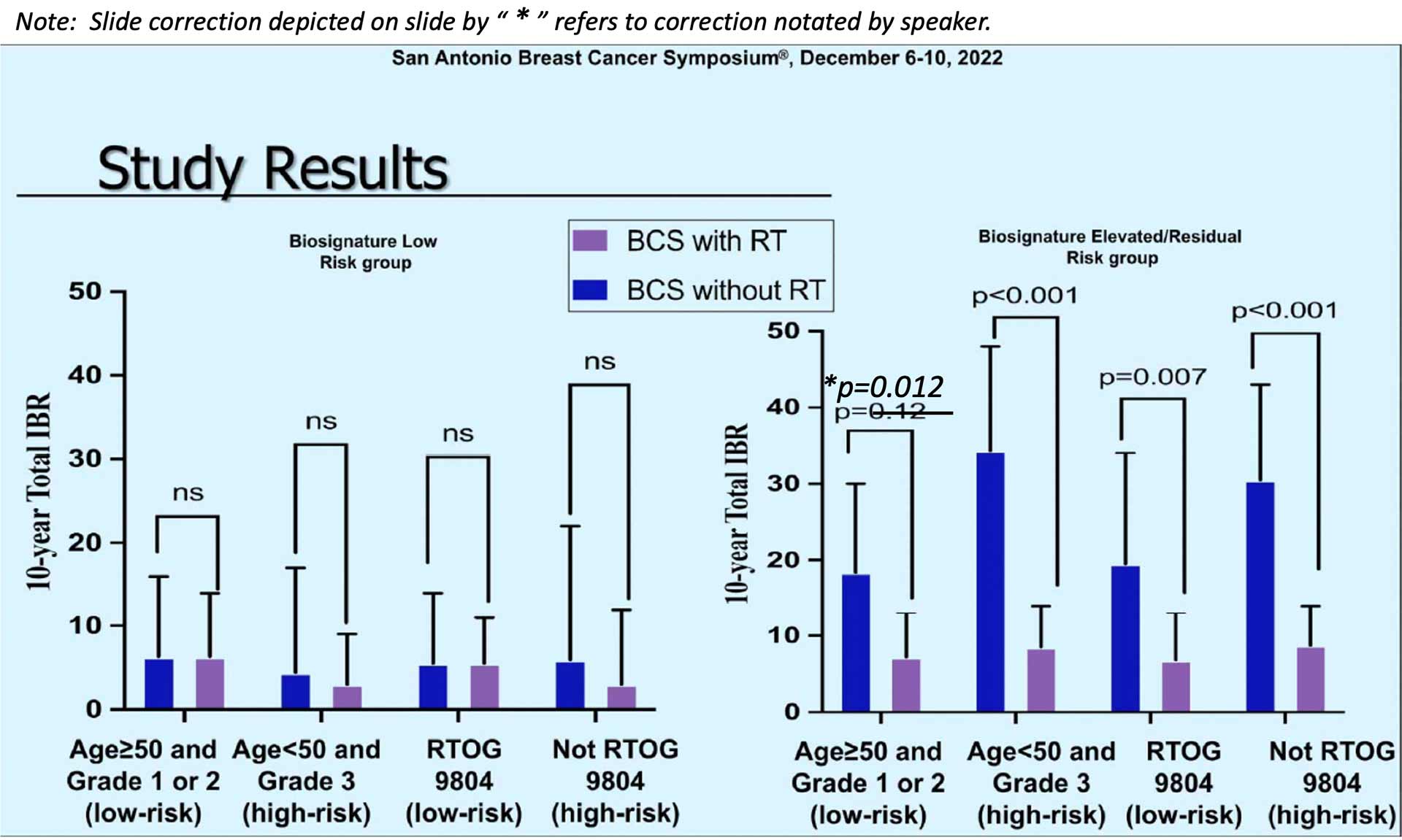
“So, biosignature in the low-risk group shows excellent outcomes and no significant radiation therapy benefit even when grade 3 or age less than 50. However, for those with an elevated-risk score or RRt there was clinically meaningful benefit with radiation even if they had favorable characteristics.”
Dr. Plitcha concluded her discussion by saying, ‘So, in your practices today, clinical pathologic factors may not be adequate for assessing radiation benefit. And the 7-gene biosignature may provide superior prediction for 10-year risk and radiation benefit than standard risk models.’
During the Q&A session, the issue of endocrine therapy and its impact on recurrence rates was addressed. Dr. Rachel Rabinovitch, Radiation Oncologist, UCHealth Radiation Oncology, Anschutz Medical Campus, confirmed ‘that the biosignature takes into account whether the margins are negative or positive. In this study, the margins were all negative’. Dr. Tony Brenner, CSO of PreludeDx, elaborated on the answer based on data that was presented at ASCO addressing the relationship between endocrine therapy following breast conserving surgery and the various risk categories. “When we looked at this within the 3 risk groups presented in the abstract, we found that the low-risk group and the residual risk group had no statistically significant risk reduction with the addition of endocrine therapy. However, the elevated-risk group saw a statistically significant risk reduction with the addition of endocrine therapy after breast conserving surgery.”

About Jennifer Plitcha, MD, MS,FACS
Associate Professor of Surgery
Associate Professor in Population Health Sciences
Member of the Duke Cancer Institute